Theories:
Thedual alkali desulphurization is a wet scrubbing technology, carried out in thetower. After reaction between NaOH and SO2, it forms a solutioninstead of slurry, which may lead to blocking. The spent caustic solutioncirculates into reaction champers, where NaOH is regenerated by Ca(OH)2.The reproductive caustic solution circulates back into the scrubbing tower. Thedual alkali desulphurization needs lower capital and operating cost. Meantime,it has good flexibility with flue gas fluctuation. Therefore, this system willbe widely applied in purifying smoke from coal-fire boiler, combustion in FCCand smoke from steel sintering.
1.Desulfurization
Theories:
The dual alkali desulphurization is a wetscrubbing technology, carried out in the tower. After reaction between NaOH andSO2, it forms a solution instead of slurry, which may lead toblocking. The spent caustic solution circulates into reaction champers, whereNaOH is regenerated by Ca(OH)2. The reproductive caustic solutioncirculates back into the scrubbing tower. The dual alkali desulphurizationneeds lower capital and operating cost. Meantime, it has good flexibility withflue gas fluctuation. Therefore, this system will be widely applied inpurifying smoke from coal-fire boiler, combustion in FCC and smoke from steelsintering.
Reactions:
Desulfurization
SO2+2Na( OH)2=Na2SO3+H2O
Na2SO3+SO2+H2O=2NaHSO3
Recycle:
2NaHSO3+Ca(OH)2=CaSO3+Na2SO3+2H2O
Features:
Dual alkali desulfurization has “3 high, 2low, 1 small”features.
1.Highdesulfurization efficiency.
2.Highutilization of alkali.
3.Highreliability.
4.Lowinvestment cost.
5.Lowrunning cost.
6.Smalloccupied area.
Process flow:

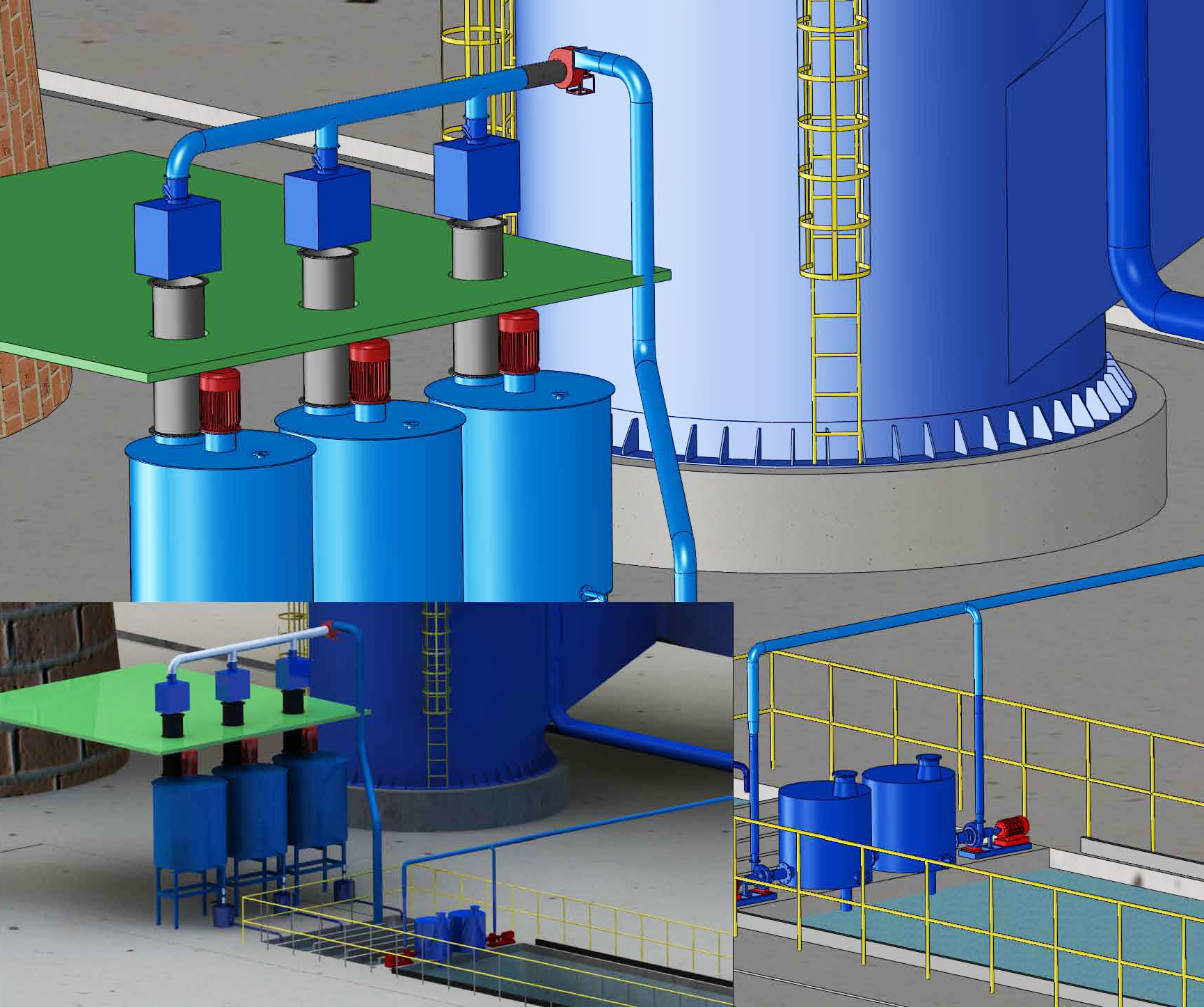
2.Limestone (CaCO3)-Lime (CaO)desulfurization
Application areas:
This technology is usedin boilers from coal fire power plant, cogeneration energy plant, centralheating system, sintering machines, coking furnaces, glass furnaces, etc.
Theories:
This method uses a slurryof alkali sorbent, limestone or lime. In case of limestone, the slurry is amixture of water and grinded limestone powder. In case of lime, the slurry isthe result of hydration of lime. In the flue gas desulfurization (FGD) tower,the flue gas fully contacts with the slurry, produces CaSO3. Then,CaSO3 is further oxidized to produce marketable CaSO4•2H2O(gypsum).
Components:
The system is consist offlue gas collecting system, absorption and oxidization system, limestone/limeslurry preparation system, by-product treatment system, waste water treatmentsystem, utility system( including water, compressed air, accident tank, etc.),electrical control system.
Reaction formulas:
Desulfurization:
CaCO3+SO2+1/2 H2O=CaSO3∙1/2H2O+CO2
Ca(OH)2+SO2=CaSO3∙(1/2)H2O+1/2H2O
CaSO3·1/2H2O+SO2+1/2H2O=Ca(HSO3)2
Oxidization:
2CaSO3∙(1/2)H2O+O2+3H2O=2CaSO4∙2H2O
Ca(HSO3)2+O2+2H2O=CaSO4∙2H2O+H2SO4
Features:
This technology has “5 high, 3 large, 2 low” features, which are listedbelow.
High desulfurizationefficiency
High operating efficiency
High reliability
High investment cost
High operating cost
Large capacity of fluegas disposal
Large capacity of SO2treatment
Large space occupied
Low Ca to S ratio
Low price of absorbent
Process flow:

京ICP备05048247号 版权所有 开元体育(中国)制造有限公司 E-mail
